ISO 9001 is a standard designed for organizations looking to optimize their operational excellence. It helps businesses and organizations to be more efficient and improve customer satisfaction. A new version of the standard (ISO 9001:2015) was launched — taking over the previous version (ISO 9001:2008).
ISO standards are reviewed every five years and revised if needed to ensure that it maintains its significance in today’s market place. This revision will also serve to bring ISO 9001 up to relevancy with regard to both challenges and opportunities that arise from changing technologies, globalization, and a reinforcement of a risk based approach, as well as structuring the standard to deal with future changes.
What are the Major Differences?
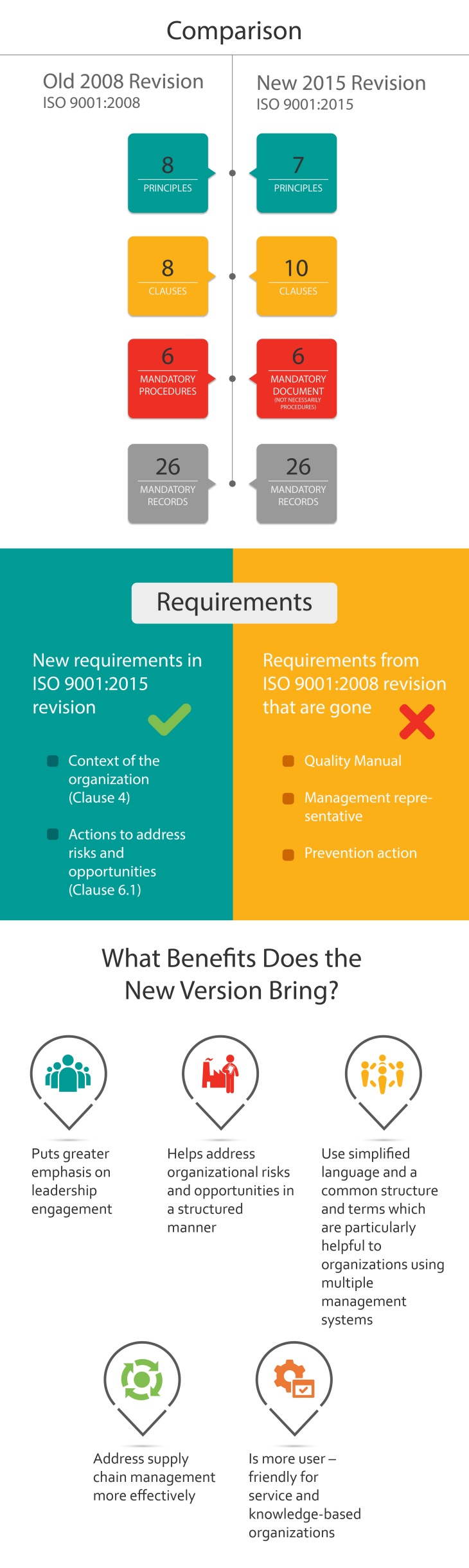
The new ISO 9001 standard aligns with high-level organizational structure, requiring all new ISO management system standards to be aligned on a high-level structure with a set of common requirements. Additionally, there is a greater emphasis on risk-based thinking as a basis for the management system, more focus on achieving value for the company and its customers, increased flexibility regarding use of documentation, and a more approachable structure for service businesses.
There are 10 clauses within the standard and here are the changes clause by clause:
Clause 1 is very similar to the 2008 version covering the scope of the standard and there has been very little change to this clause.
Clauses 2 and 3 cover normative references and term and definitions, both these clauses reference ISO 9000, Quality Management System – Fundamental and vocabulary which provides valuable guidance.
The remainder of the clauses includes some new key elements which need to be considered when implementing the new standard.
Clause 4: Context of the Organization
This is a new clause that in part addresses the depreciated concept of preventive action and in part establishes the context for the QMS.
Clause 5: Leadership
This clause places requirements on top management to demonstrate commitment to the QMS through taking accountability for the effectiveness of the QMS, establishing policies, objectives and promotion of continual improvement.
Clause 6: Planning
When planning the QMS, the organization will need to consider the external and internal issues along with needs and expectations of interested parties.
Clause 7: Support
The organization shall determine and provide the necessary resources to establish, implement, maintain and continually improve the QMS.
Clause 8: Operation
This clause deals with the execution of the plans and processes that enables organization to meet their quality policy and quality objectives.
Clause 9: Performance Evaluation
This clause sublimates all requirements for monitoring and measurement related to quality performance and effectiveness of their QMS.
Clause 10: Improvement
The organization must determine the opportunities for improvement to continually improve the organization’s QMS.
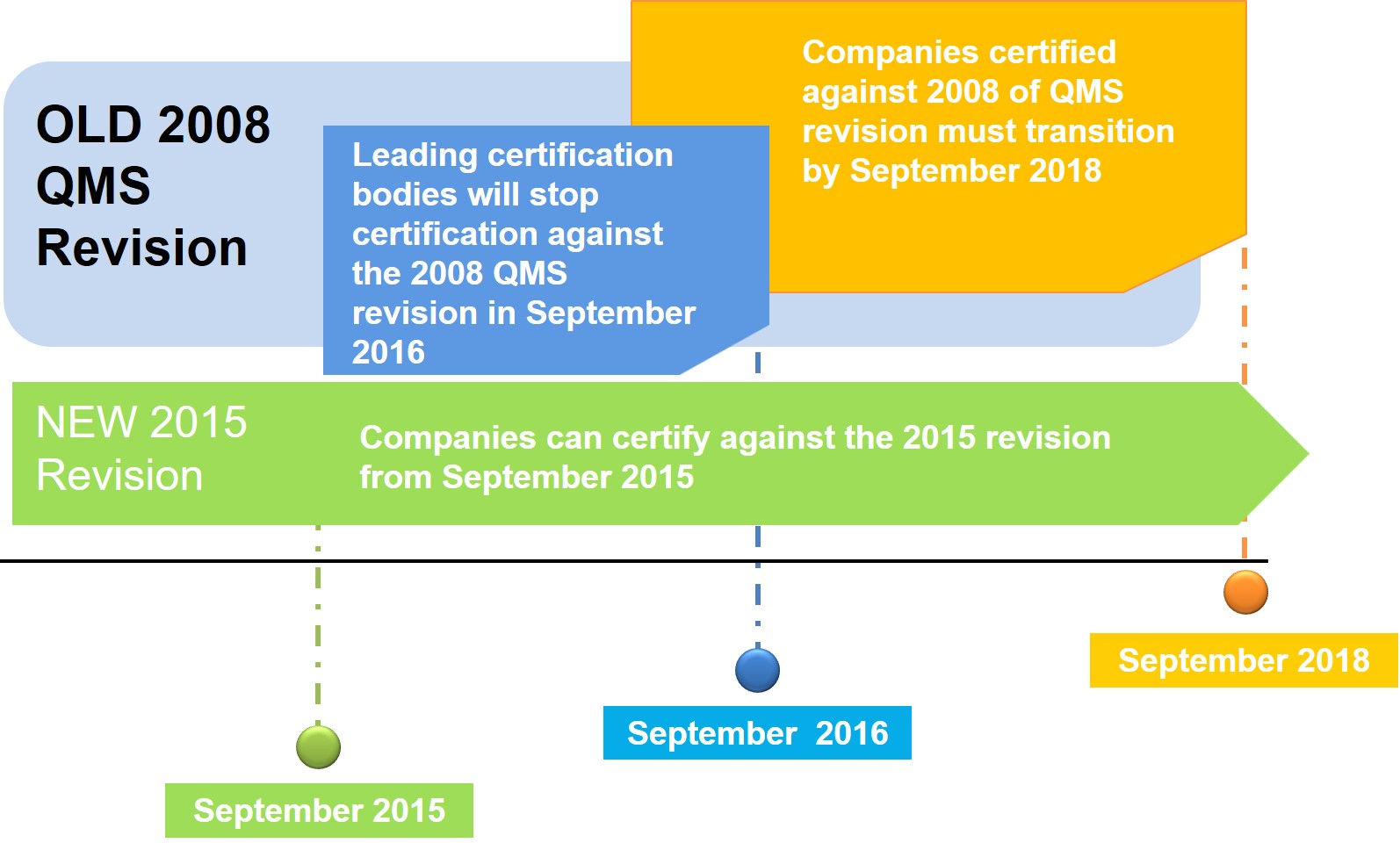
Impact of the New Standard
ISO 9001:2015 is now taking off to replace ISO 9001:2008. Organizations who are already ISO 9001 certified should begin tracking their progress of the revision process and familiarize themselves with the various changes made. To maintain your certification to ISO 9001, you will need to upgrade your quality management system to the new edition of the standard and seek certification to it. You have a three-year transition period from the date of publication (September 2015) to move to the 2015 version. This means that, after the end of September 2018, a certificate to ISO 9001:2008 will no longer be valid.
According to the International Accreditation Forum (IAF), there are a number of recommended actions that organizations can take to successfully transition to the new requirements of ISO 9001:2015. These include:
- Conduct a gap analysis
Identifying the gaps between current practices and the new requirements is the most effective way to evaluate the changes that are required in your current QMS.
- Develop an implementation plan and timetable
A formal implementation plan and schedule will help your organization address the required changes within the anticipated three-year transition period.
- Provide appropriate training for all parties
Ongoing education and training for all relevant personnel are critical to achieving the goals of your transition plan. More important, educated stakeholders are vital in ensuring ongoing compliance once the transition is complete.
- Update existing QMS documentation
Clear and thorough documentation is essential to demonstrate compliance with the requirements of the revised standard and to help reduce the risk of nonconformities.
- Involve your certification partner early in the process
An experienced certification body can provide invaluable assistance in the process of transitioning to the requirements of ISO 9001:2015. Its early involvement can help your organization save time and money.
Conclusion
In a nutshell, there are new areas that organization need contemplate in the implementation of the new standard, but it provides opportunity to review your current approach and modify it if necessary. This can help your business to grow, increase profitability and increase customer satisfaction. It is now a powerful business improvement tool for all sizes and types of organizations to help them remain irrepressible and achieve sustainable growth.
Are you ready for the transition?
Let us assist you!
Sources:
http://www.iso.org/iso/iso_9001_-_moving_from_2008_to_2015.pdf
http://www.qualitydigest.com/inside/quality-insider-article/080515-iso-90012015-avoiding-nonconformities-during-transition.html
http://www.qualitymag.com/articles/92754-the-new-iso-90012015-why-its-still-relevant-and-what-are-the-changes